Several scanning modalities are used to investigate and detect skeletal abnormalities. Most commonly, one finds in clinical practice low-dose CT which is used to detect deviations from normal bone structure, MRI to detect bone marrow and other soft tissues abnormalities as well as PET CT to investigate into the metabolic activity of suspected tissues. To reduce the cumulative exposure risk, clinical practice has recently been shifted toward whole-body imaging. However, resolution in such images and the low dose used render complete localization of suspected regions difficult. Precise and automated methods, able to improve the ability of an expert to pinpoint abnormalities, are thus called for.
Quantification of primary and metastatic bone cancer
With the advancement of image processing and computer vision methodologies, an alert system can be constructed by algorithmic means. Such systems are designed to automatically localize metastatic and primary cancerous regions. Quantitative characterization of the disease can then enable oncologists to better assess prognosis, which should then translate into personalized treatment, higher survival rate and improved quality of life.
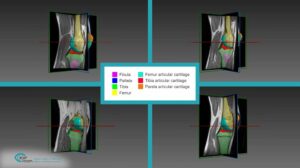
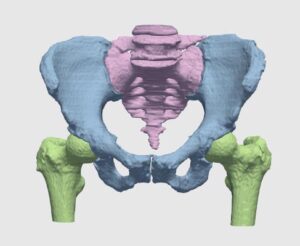
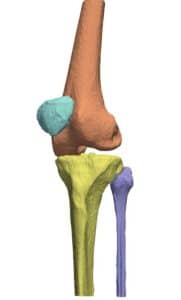
The detection procedure begins with skeletal segmentation and its distinction from surrounding anatomical parts. For this end, incorporation of knowledge into the anatomical part is useful to determine sensitivities related to bone density and relative intensity as seen by the image modality. In whole-body imaging, it is advisable to distinguish body parts and perform segmentation on each part. By itself, the recognition of body parts is not trivial. However, owing to the predicted patient pose at the time of imaging, coupled with computational models of human anatomy, such a task is indeed feasible.
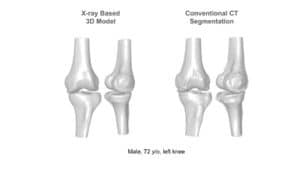
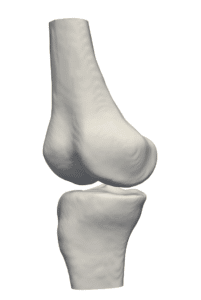
The availability of successive stacked 2D images enables iterative segmentation to produce 3D surfaces of bones and other skeletal-related structures. Stitching 2D slices accurately into a 3D model requires a primary registration and matching step to align slices and fix their relative position to other organs. Methodologies for 3D reconstruction are widely used in CAD/CAM for computer assisted surgery (CAS) and provide oncologists with a more complete image of the skeletal structure, to assist in quick detection of structural anomalies.
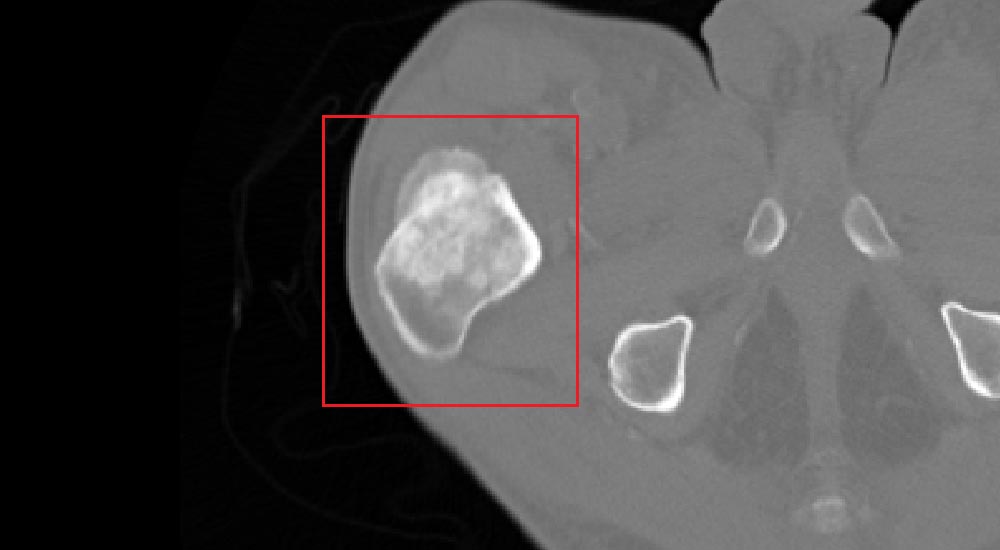
Metastatic lesions detection is performed on the segmented skeletal structure in two and three dimensions. The challenge faced by algorithms is to simultaneously extract the geometrical properties of the lesions and classify candidate structures into their severity levels. Classification of structures can be successfully performed by utilizing Support Vector Machine-based (SVM) algorithms, which ‘learn’ by way of supervision the characteristics of a set of normal and abnormal manually classified lesions. Learning process can be regarded as synonymous to adjusting weights of the SVM, once the set enables autonomous classification of newly encountered suspected structures.
The pipeline shortly outlined above by no means describes all challenges to be faced during the construction of such algorithms. In light of the accumulative information of patient history, prognostic factors and multimodal data, algorithmic means seem to be the only reasonable way to coherently incorporate all this info into the diagnosis and treatment planning. Rich of its long experience in automatic detection, 3D reconstruction and multimodal registration, RSIP Vision can help you succeed in all your algorithmic projects.

 Orthopedics
Orthopedics